electrons of calcium|how many protons in calcium : Baguio Calcium is the 20th element in the periodic table. It is a group 2 metal, also known as an alkaline-earth metal, and no populated d-orbital electrons. Calcium is the .
Free Slots Real Money Slots; Purpose: To provide a fun and risk-free way of playing slot games. To provide a chance to win real money playing slot games. Risk: No financial risk as no real money is wagered. There’s a financial risk as you need to bet real money. Gameplay: Simulates real slot games. Exact real-world slot game .
PH0 · valence electrons of calcium
PH1 · how many protons in calcium
PH2 · ca protons neutrons electrons
PH3 · Iba pa
Nude Leaks . ICLOUD LEAKS 2024; Home; Browse; Emma Watson; Jennifer Lawrence; Catryona Lei / Ryona Lei Perez / _ayecatxryona Nude OnlyFansPAGCOR Chairman Alejandro H. Tengco and Thomas Arasi - President and Chief Operating Officer, Bloomberry Resorts Corp., headline a stellar speaker lineup at the 6th edition of the ASEAN Gaming Summit on 19th-21st March 2024.
electrons of calcium*******These blocks are named for the characteristic spectra they produce: sharp (s), principal (p), diffuse (d), and fundamental (f). Atomic numberThe number of protons in an atom. Electron configurationThe arrangements of electrons above the last (closed shell) noble gas.The arrangements of electrons above the last (closed shell) noble gas. Melting .Potassium salts in the form of saltpetre (potassium nitrate, KNO 3), alum .
Calcium was named after the Latin term calx meaning lime, and is a reactive .

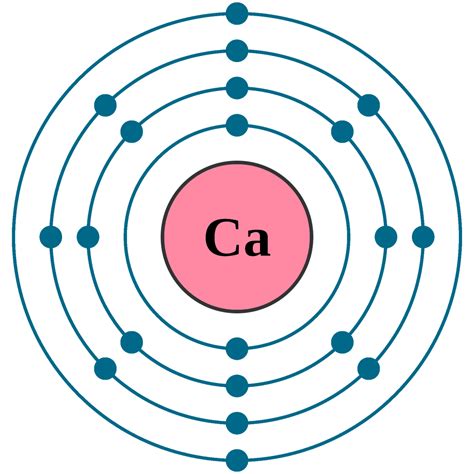
Calcium is a very ductile silvery metal (sometimes described as pale yellow) whose properties are very similar to the heavier elements in its group, strontium, barium, and radium. A calcium atom has twenty electrons, with electron configuration [Ar]4s . Like the other elements placed in group 2 of the periodic table, calcium has two valence electrons in the outermost s-orbital, which are v.
Calcium is the 20th element in the periodic table. It is a group 2 metal, also known as an alkaline-earth metal, and no populated d-orbital electrons. Calcium is the .
Stable Isotopes. Electrons and Electron Configuration. The number of electrons in an electrically-neutral atom is the same as the .
In order to write the Calcium electron configuration we first need to know the number of electrons for the Ca atom (there are 20 electrons). When we write the configuration .
Electrons: 20: Protons: 20: Neutrons in most abundant isotope: 20: Electron shells: 2,8,8,2 : Electron configuration: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2: Density @ 20 o C: . Calcium from limestone is a vital component of .
Calcium is the 20th element in the periodic table and has a symbol of Ca and atomic number of 20. It has an atomic weight of 40.078 and a mass number of 40. Calcium has .electrons of calcium how many protons in calciumThe Electron configuration of calcium. Its electron configuration is as follows: 1s22s22p63s23p64s2. It has a total of 20 electrons, which are distributed as follows: 2 .Electron configuration of Calcium is [Ar] 4s2. Possible oxidation states are +2. Density of Calcium is 1.55g/cm3. Typical densities of various substances are at atmospheric pressure. Density is defined as the mass .Atomic Number – Protons, Electrons and Neutrons in Calcium. Calcium is a chemical element with atomic number 20 which means there are 20 protons in its nucleus.Total number of protons in the nucleus is called the .
What is the Electron Configuration of Calcium. Calcium’s atomic number is 20 which means that in a neutral calcium atom, in its nucleus there are 20 protons. The electron configuration of a Ca ion is .
how many protons in calciumThe 20 th element in the periodic table is calcium. Calcium is an alkaline earth metal and its symbol is ‘Ca’. Calcium participates in the formation of bonds through its valence electrons.. This article discusses in detail how .The Electron configuration of calcium is 1s22s22p63s23p64s2. Calcium is one of the chemical elements of the periodic table considered an alkaline earth metal, belonging to group 2, period 4 and the s block of it. It is distinguished from other elements by its symbol Ca and by its atomic number, which is 20. At the same time, it has an atomic . In this video we'll look at the atomic structure and Bohr model for the Calcium atom (Ca). We’ll use a Bohr diagram to visually represent where the electrons.
To illustrate, an atom of an alkali metal (group 1) loses one electron and forms a cation with a 1+ charge, as presented in Figure \(\PageIndex{1}\); an alkaline earth metal (group 2) loses two electrons and forms a cation with a 2+ charge, and so on. For example, a neutral calcium atom, with 20 protons and 20 electrons, readily loses two .The atomic mass number is represented by A, so, for Calcium A = 40. To calculate the number of protons, electrons, and neutrons: The number of protons: The number of protons present in an atom is equal to the atomic number of the element. Thus, in Calcium there are 20 protons are present.An atom's valence electrons are the electrons in its outermost shell. In the chlorine model below, the valence electrons are shown in red . The number of valence electrons determines most of an atom's chemical behaviors. So, it's important to be able to identify how many valence electrons atoms of different elements have. Fortunately, we can .Each element's electron configuration, which was determined in the previous section, is shown below. Neon; 1 s 2 2 s 2 2 p 6. Calcium; 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 4 s 2. Answer a Neon has electrons in the first and second energy levels, as indicated by the leading red 1 and 2 's, respectively. Valence electrons are those found in the highest . Calcium-40 is a stable isotope containing 20 neutrons. 96.941% of natural calcium is calcium-40. Calcium-40 is theorized to actually be a radioactive isotope with an extremely long half-life (~10 21 years) based on its internal structure. No one has ever detected a decay of a calcium-40 atom. 42 Ca. In this video we’ll use the Periodic table and a few simple rules to find the protons, electrons, and neutrons for the element Calcium (Ca). . and neutrons for the element Calcium (Ca). From . Valence electrons: For main group elements (i.e s-block and p-block elements), the valence electrons are the electrons present in the outermost orbit. But for most of the transition and inner transition . Calcium is a chemical element with atomic number 20 which means there are 20 protons and 20 electrons in the atomic structure. The chemical symbol for Calcium is Ca. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. The nucleus is composed of protons and neutrons. Step 1: Write the electron configuration of the atom in the following form: (1s) (2s, 2p) (3s, 3p) (3d) (4s, 4p) (4d) (4f) (5s, 5p) . . . Step 2: Identify the electron of interest, and ignore all electrons in higher groups (to the right in the list from Step 1). These do not shield electrons in lower groups. Step 3: Slater's Rules is now broken .Well, calcium's electron configuration, I could do it in noble gas notation or configuration, it'd have the electron configuration of argon, and one of the reasons why the noble gases are so stable is that they have a completely full shell. Argon for example has a completely full first shell, second shell, and third shell, and then to build . The metal itself is used as an alloying agent for aluminum, copper, lead, magnesium, and other base metals; as a deoxidizer for certain high-temperature alloys; and as a getter in electron tubes.Small percentages of calcium are used in many alloys for special purposes. Alloyed with lead (0.04 percent calcium), for example, it is employed .
electrons of calciumFor instance, the ground state electronic configuration of calcium (Z=20) is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2. The calcium ion (Ca 2+), however, has two electrons less. Hence, the electron configuration for Ca 2+ is 1s 2 2s 2 2p 6 3s 2 3p 6. Since we need to take away two electrons, we first remove electrons from the outermost shell (n=4). Electron configuration chart of all Elements is mentioned in the table below.The Shorthand electron configuration (or Noble gas configuration) as well as Full . Electron configuration of Calcium (Ca) [Ar] 4s 2: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2: 2, 8, 8, 2: 21: Electron configuration of Scandium (Sc) [Ar] 3d 1 4s 2: 1s 2 2s 2 2p 6 3s 2 3p 6 3d 1 .
High Explosive: Directed by Barry Crane. With Larry Wilcox, Erik Estrada, Robert Pine, Steve Oliver. Ponch and Jon befriend a fatherless country boy out of place in the big city and in big trouble for firing his pellet gun into traffic. A reckless ambulance driver threatens lives when he illegally transports cases of unstable dynamite.
electrons of calcium|how many protons in calcium